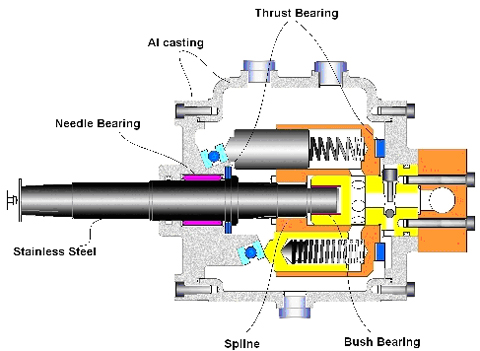
Replacing the boat engine with a battery-powered hydraulic system
A hydraulic system is a type of motor that uses a fluid to transmit power from a motor to a load. It is used in industrial applications and in most…
How the Marine Engineering is Disrupting Manufacturing
Marine engineering is a new industry that has the potential to disrupt manufacturing. It is a relatively new industry and there are many aspects of it that are still unclear….
A Beginner’s Guide to Marine Engineering
Marine engineering (ME) is the application of engineering principles and practices to marine structures and systems. ME is a broad field that includes everything from designing ships to designing underwater…